Understanding Hyperbaric Oxygen Therapy (HBOT)
WITHOUT HYPERBARICS
WITH HYPERBARICS
Hyperbaric Oxygen Therapy (HBOT) is a pain-free and non-invasive treatment that addresses multiple medical conditions. HBOT involves breathing 100% pure oxygen inside a pressurized chamber with the help of a mask over the face. The patient breathes over 10 to 20 times more oxygen, then carries along with the blood throughout the body, reaching the wounded and other infected areas. The oxygen diffuses into blood plasma as the patient breathes 100% oxygen. Thus, helping reduce discomfort and swelling and repairing the damaged tissue. An HBOT session usually continues for about 60 to 90 minutes.
Research and clinical studies on the effectiveness of HBOT are carried out now and then for various medical conditions. HBOT improves and enhances your health by helping your body repair, regenerate, and heal. It can also help restore normal function and relieve chronic pain.
Hyperbaric Oxygen Therapy is similar to carbon dioxide (CO2) compressed in a soda bottle. The CO2 bubbles, when under pressure, reduce in size, unseen to us. However, with the release in pressure, the bubble size increases, becoming visible. Similarly, when the patient is under pressure inside the HBOT chamber, the size of the oxygen molecule decrease and is dissolved in the blood plasma. The increase in oxygen in the body helps it reach the damaged tissues, thus improving the ability to regenerate and support the functioning.
Hyperbaric Therapy and Its History
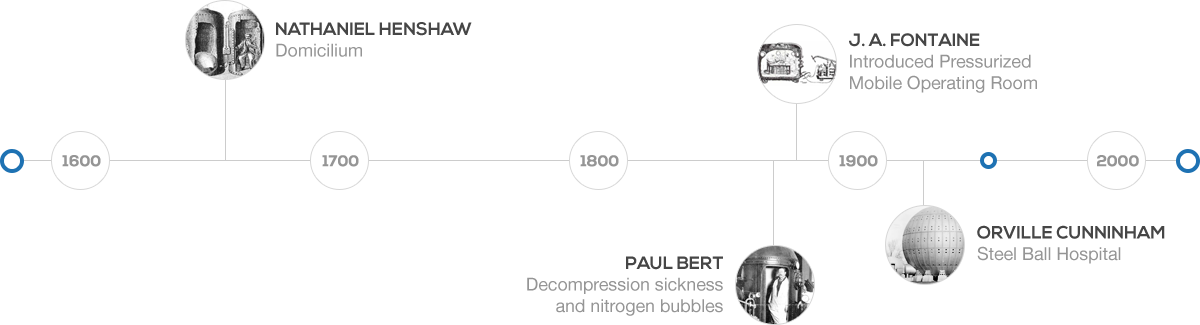
HBOT therapy is not a newly discovered treatment but takes us back to the 1600s. It was 1662 when a British physician named Nathaniel Henshaw created a chamber named Domicillium. He believed that patients with lung or digestion problems could benefit under a pressurized condition. French physiologist Paul Bert in the year 1878 uncovered the connection between nitrogen bubbles and decompression sickness. Hyperbaric chambers were in use by 1877. It was Fontaine, a French surgeon to develop the portable operating room within the pressurized chamber in 1879.
During the early 1900s, an anesthesia professor, Dr. Orval Cunningham, worked with patients suffering from cardiovascular disease and found that those living at sea level suffer less than those at high altitudes. Cunningham believed that patients under greater air pressure differences would benefit more. Eventually, the professor designed a chamber of 27 meters and started successfully treating multiple medical conditions.
In the 1930s, the US military used hyperbaric treatment to treat navy divers suffering from decompression sickness. In the 1950s, physicians employed the hyperbaric treatment for the first time during heart and lung operations. This led to HBOT being utilized for carbon monoxide poisoning. After thousands of clinical trials, research, and studies, experts found that hyperbaric treatment is beneficial for numerous health-related issues with the majority of the results resonating with success.
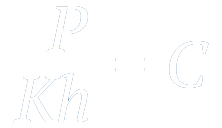

"At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid."
Here,
C - the solubility of a gas in a solvent at a fixed temperature
k - Henry's law constant
P - Partial pressure of the gas
Pressure is needed to properly dissolve the gas in a liquid form, i.e., oxygen into the blood plasma. Hyperbaric environments have higher oxygen levels than standard conditions, which help advance into deeper tissues.
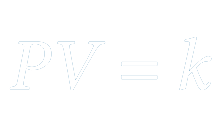

The pressure exerted by a gas is inversely proportional to the volume occupied by it if the amount of gas and the temperature remain unchanged.
Here,
P - pressure
V - volume
k - constant
With the increase in pressure, the volume of oxygen molecules reduces, making a denser oxygen territory. The oxygen molecules within the alveolus elevate, allowing the oxygen to be transported to the blood.
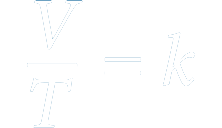

The volume of a given mass of gas is directly proportional to the absolute temperature of the gas when pressure and volume are constant.
Here,
V - Volume of the gas
T - Temperature of the gas
k - A non-zero constant.
The temperature inside the hyperbaric chamber increases with the increase in pressure. This increase in the temperature directly affects the gas volume, which increases the magnitude of the oxygen.
